ABSTRACT
Treatment of hydrocephalus in childhood remains a major challenge, due to shunt complications and the failure of the third ventriculocisternostomy (ETV) in many cases. The addition of choroid plexus coagulation (CPC) seems to open a new perspective in the endoscopic treatment of hydrocephalus. The author intends to share his personal experience in the treatment of hydrocephalus by ETV and CPC using a rigid endoscope. Technical aspects before, during and after surgery will be presented and commented on. Despite some controversies, the international literature and the author’s experience have shown that the addition of choroid plexus coagulation to the third ventriculocisternostomy can improve results in selected cases of hydrocephalus even with the use of rigid endoscopes.
KEYWORDS: Hydrocephalus, Endoscopy, Choroid Plexus, Coagulation.
INTRODUCTION
Treatment of hydrocephalus in childhood is a major challenge in modern pediatric neurosurgery, especially as it is a complex and variable disease related to etiological and pathophysiological aspects. We can affirm that the different forms and technologies of shunt derivations, while saving children also mean another disease, often as lethal as the underlying disease. Infectious and mechanical complications increase treatment costs and morbidity and mortality (01).
The appearance of new endoscopic technologies and advances in video systems gave hope that an internal derivation could resolve the hydrocephalus without the implant of shunts. However, depending on age and etiology, ETV has not achieved good results (01).
Since 1918, CPC was already known as a surgical technique aimed at the treatment of hydrocephalus, but the results were catastrophic and the technique was abandoned. In the 70s, with technological improvement, CPC by the endoscopic route was again considered in order to decrease the production of CSF. Pople and Griffith published a series of endoscopic CPC cases at the end of 1993 with only 33% good results and with many complications (03).
In 2005, Benjamin Warf starts a series of publications reporting his experience in the African continent, obtaining good results in the association of ETV with CPC. He achieved good results in 65% of children under 6 months of age and in 81% of children under 1 year. Infectious complications were recorded at 1%. (05).
Zandian et al, published a meta-analysis in 2014, comparing ETV with ETV/CPC groups in 10 studies.. Success rates were higher for ETV/CPC in patients with hydrocephalus associated with haemorrhages, infections, Dandy-Walker malformation and neural tube defects, especially in those over 2 years old (84%) (06). Chamiraju (2014), in her series of 27 premature patients with post-haemorrhagic hydrocephalus, ETV/CPC was a success in 37% of the cases (07). Weil et al. (2015) published the result of a cohort involving 85 patients with less than 2 years of life. In all cases, a rigid endoscope was used and ETV/CPC was performed. The results were considered successful (08). Pedrosa at col. published in 2017 a series of 42 patients with extreme hydrocephalus or hydranencephaly treated with endoscopic CPC. They considered the procedure effective at 70.6% (08, 01).
Some authors argue that the flexible endoscope is indispensable to reach the choroid plexus in all its extension. However, in most published reports rigid neuro endoscopes were used. Fallah et al. (2017) presented 84 cases of ETV/CPC, in which the cauterization was subtotal in 69% and partial in 31%, and found no statistically significant differences in the results (10).
PRE-OPERATIVE IMAGE ACQUISITION
Magnetic resonance imaging is the best alternative to visualize the structures of the central nervous system. The FIESTA sequences can provide important information about the third ventricle floor, cerebral aqueduct and the presence of fibrosis and adhesions in the pre-pontine cistern.
OPERATING TECHNIQUE
The indispensable equipment: neuro endoscope; endoscopic tweezers and scissors; high definition video equipment; Forgarty catheter N ° 3 or N ° 4; endoscopic monopolar, bipolar or LASER; and, if available, trans-operative ultrasonography and neuronavigation.
Short and thinner endoscopes are recommended in newborns and infants. In macrocranias longer endoscopes are recommended.
The use of bipolar is more efficient than the monopolar. Take care about intensity of the coagulation because propagation of heat and the heating of CSF. Continuous irrigation of the ventricles must be assured with control of temperature. Contact LASER allows a more efficient coagulation, with less heat and detritus productions.
Neuronavigation can be of great help in the planning and orientation during approach and trans-operatory ultrasonography allows real-time images.
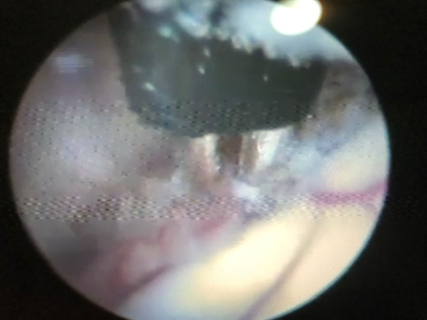
The patient should be positioned in dorsal decubitus with a slight anterior flexion of the head. This can minimize the formation of pneumencephalus. The head should be well fixed. Asepsis and preparation of the operative field. Incision of the skin 1,5 – 2 cm from the midline, at the level of the coronary suture. In infants trepanation is not necessary. Careful cross-cut or straight incision of the dura mater can be made. In older children, trepanation, haemostasis with bone wax is necessary before dura incision. After coagulation of the dura mater and adjacent cortex, the endocospe should be introduced slowly and carefully, guided by the neuronavigation and the video system. Introduction of the endoscope in the lateral ventricle. Identification of the choroid plexus. The ETV should be performed first. Following the choroid plexus in the postero-anterior direction we find the foramen of Monro. We cross the foramen of Monro and we are in the third ventricle. Between the mamillary bodies, superiorly, with the aid of a Forgaty catheter, we gently puncture the floor of the third ventricle, filling the balloon to enlargement of the orifice. The Lilliquist’s membrane should be opened. CPC must be performed in the craniocaudal direction, extensive as possible, coagulating the nourishing artery that accompanies the choroid plexus without injury to adjacent brain structures (Figure 1, 2 and 3).
Figure 1: CPC with bipolar forceps.
Figure 2 : Continuation of right side CPC.
Figure 3: Coagulated left choroid plexus.
The bilateral approach is facilitated in cases of single ventricle, absence of septum or fenestrated septum. Having a normal septum, a septostomy may be performed or the contralateral ventricle may be approached through a new trephination. Small bleeding can be controlled with Ringer’s solution irrigation.At the end, after the endoscope is removed, be careful with the hermetic closing of the dura-mater to avoid CSF fistula. External drainage catheter monitoring is recommended and surveillance in an intensive care unit for 48 – 72 hours. The author considers as surgical success the cases in which no other procedure is necessary.
CONCLUSIONS
Despite the existence of some controversies and the need for further investigation on the indications and long-term effects, the international literature and the author’s experience have shown that the addition of CPC to ETV can improve the treatment of hydrocephalus, even with the use of a rigid endoscope.
The author declares that he has no conflict of interest related to this article.
BIBLIOGRAPHIC REFERENCES.
- Da Cunha A H G B. Coagulation of The Choroid Plexus. In: Dabdoub Arrien C, Editor. Neurocirugia Infantil Latinoamericana, Tomo 3, Primera Edición. Santa Cruz de la Sierra: Editorial UNIFRANZ; 2018: 237 – 246.
- Zhu X, Di Rocco C. Choroid Plexus Coagulation for Hydrocephalus not due to CSF Overproduction: a review. Child´s Nerv Syst. 2013:29:35-42.
- Pople I K, Griffith H B. Control of Hydrocephalus by Endoscopic Choroid Plexus Coagulation-lonmg-term Results and Complications. Europ Journal of Pediatric Surgery, 1993; Suppl 1:17-8.
- Warf B C. Comparison of Endoscopic Third Ventriculostomy Alone and Combined with Choroid Plexus Cauterization in Infants Younger tan 1 Year of Age: A Prospective Study in 550 African Children. J Neurosurgery. 2005 Dec; 103(6 Suppl); 475-81.
- Warf B C. Congenital Idiopathic Hydrocephalus of Infancy: The Result of treatment by endoscopic third ventriculostomy with or without Choroid Plexus Cauterization and Suggestions for how it Works. Child´s Nerv Syst. 2013 Jun;29(6):935-40.
- Zandian A, Haffner M, Johnson J, Rozzelle C J, Tubbs R S and Loukas M. Endoscopic Third Ventriculostomy with/without Choroid Plexus Cauterization for Hydrocephalus Due to Hemorrhage, Infection, Dandy-Walker malformation, and Neural Tube Defect: A Meta-analysis. Child´s Nerv Syst. 2014; 30:571-578.
- Chamiraju P, Bhatia S, Sandberg D I, Ragheb J. Endoscopic Third Ventriculostomy and Choroid Plexus Cauterization in Posthemorrhagic Hydrocephalus of Prematurity. J Neurosurg Paediatric. 2014 Apr; 13(4):433-9.
- Weil A G, Fallah A, Chamiraju P, Ragheb J, Bhatia S. Endoscopic Third Ventriculostomy and Choroid Plexus Cauterization with a Rigid Neuroendoscope in Infants with Hydrocephalus. J Neurosurg Pediatric. 2015 Oct; 30:1-11.
- Pedrosa H A R, Lemos S P, Vieira C et all. Choroid Plexus Cauterization on treatment of Hydranencephaly and Maximal Hydrocephalus. Child´s Nerv Syst. 2017 Sep; 33(9):1509-1516.
- Fallah A, Weil A G, Juraschka K et all. The Importance of Extend of Choroid Plexus Cauterization in Addition to Endoscopic Third Ventriculostomy for Infantile Hydrocephalus: A Retrospective North American Observational Study Using Propensity Score-Adjusted Analysis. J Neurosurgery Paediatric. 2017 Dec; 20(6):503-510.